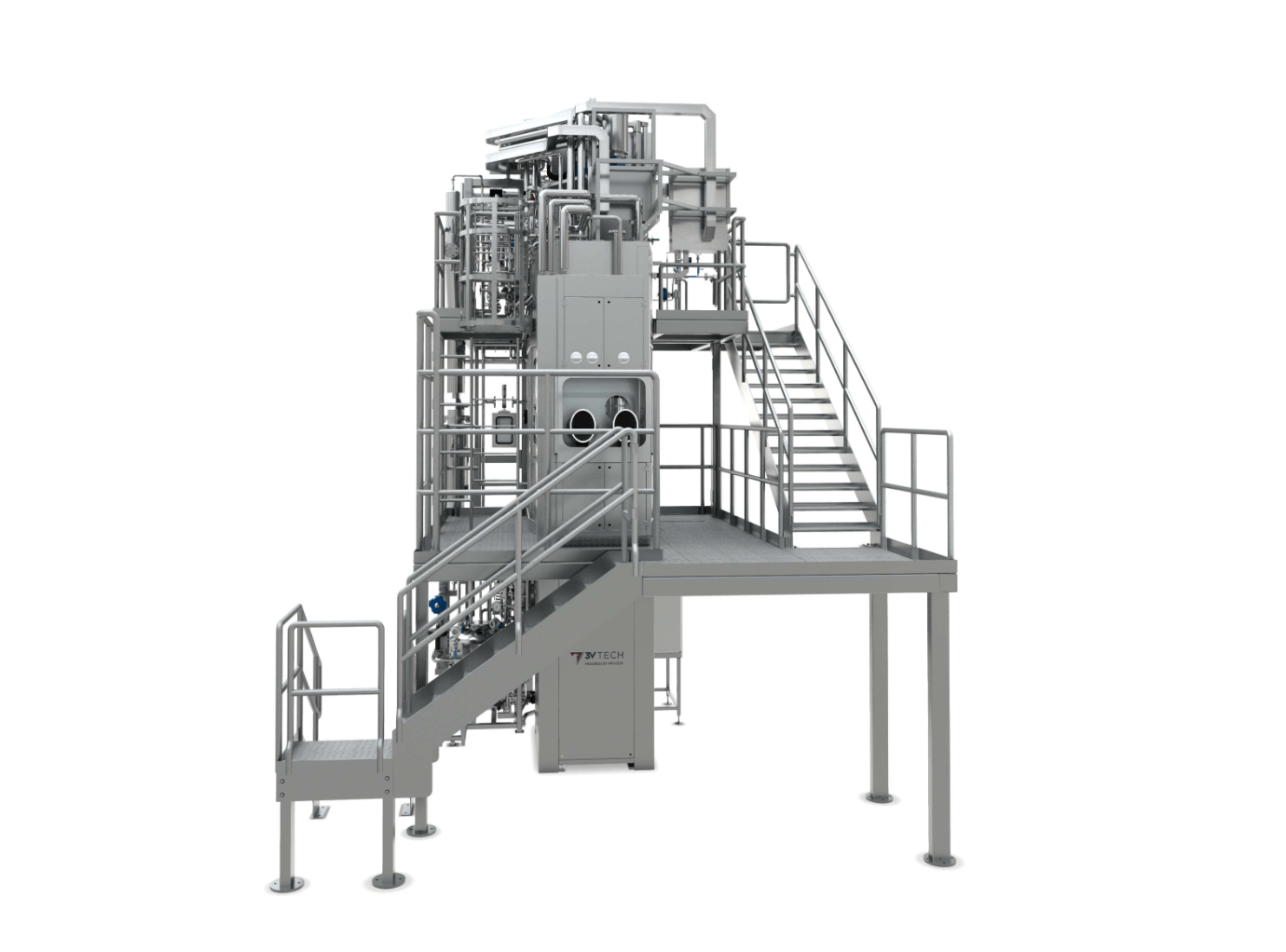
Plants
Modular systems for API, HPAPI and intermediates filtration & drying
The synthesis of active pharmaceutical ingredients (APIs), essential substances used in drug products to obtain a therapeutical effect, is a multi-step chemical process consisting in transforming raw starting materials into several intermediates until the final molecule is obtained.
Each step may represent a manufacturing process itself requiring multiple units in addition to the need, especially for HPAPIs (highly potent active pharmaceutical ingredients), to use containment equipment to ensure process quality and safety.
To fully support our customers, we provide a full range of modular turnkey systems for different process and operation units:
A single specific module or multiple modules can be provided based on the project’s complexity.
Overview
Show more Show lessOur filtration and drying systems may include:
- A dryer or filter dryers
- Containment systems (milling, packing, weighing)
- Interconnecting piping
- Valves & instrumentation
- A supporting structure
- A walking platform and access ladders
The following ancillary equipment is also provided on request:
- Automation package
- Vacuum group
- Heating and cooling systems
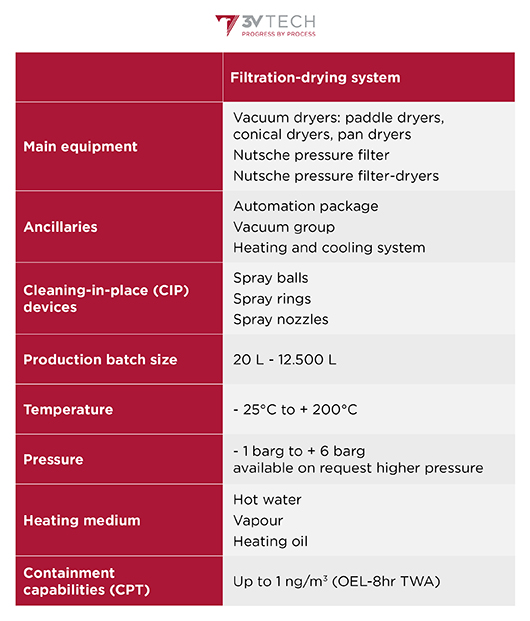
Features and rangeability
Show more Show lessCustomised systems are supplied according to a customer’s needs and expectations; typical features are reported below.
Should a customer so request, systems can be provided that comply with (or that don’t comply with) the following industry standards:
- GMP
- ATEX
- Processing in an aseptic environment for the production of sterile drugs
In addition to plant systems and equipment, we offer a wide range of services, from technical feasibility studies to installation and start-up and, cognizant of the attention pharmaceutical industries have for the environment, we also develop and supply solutions to treat wastewater and improve discharge quality.
Key benefits
Show more Show less- More efficient project management thanks to less suppliers
- Decreased project costs and schedule time
- Fully integrated services from process design to construction, installation and start-up
- Customised design
- High automation level
- Remote assistance
Applications
Show more Show lessModular systems can be provided to produce different kinds of intermediates, APIs and HPAPIs used for oral solid dosage as well for injectables, parenteral formulations (sterile) and diverse reactions, including cryogenic ones.
Process parameters and equipment need to be specifically identified for each application to minimise development times and costs, as well to support process scale-up. We conduct laboratory and pilot testing for filtration and drying steps, leveraging the capabilities of our R&D centre and multidisciplinary teams.
References